Unbelievable Tips About How To Increase Rate Of Reaction

Investigate factors which affect the speed of a chemical reaction and calculate the time taken for the reaction to occur in national 5 chemistry.
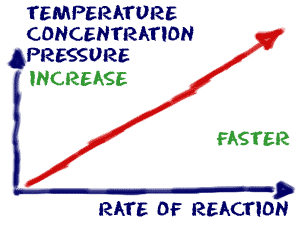
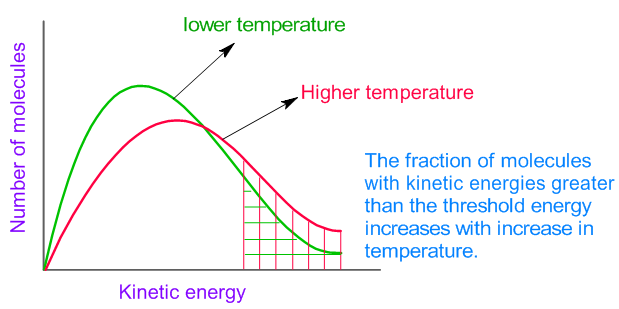
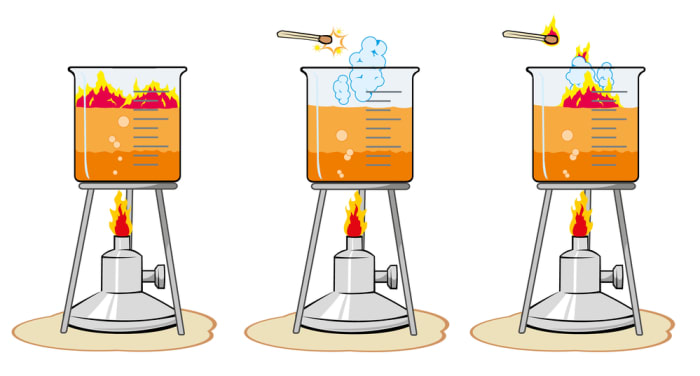
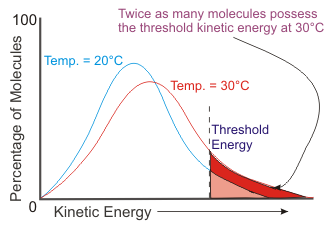
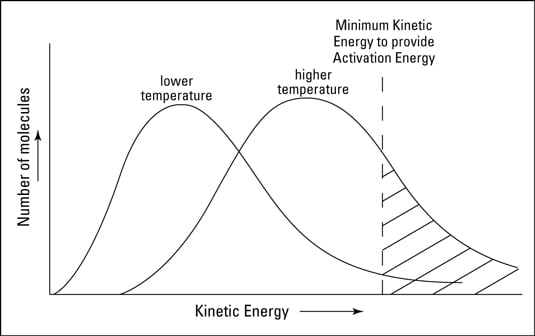
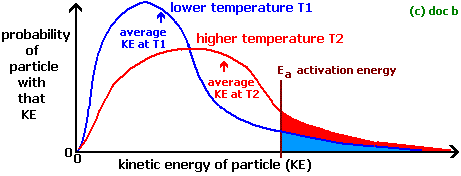
How to increase rate of reaction. Concentration (of solutions) surface area, concentration and pressure all have the same effect on reaction rate (an increase leads to a faster reaction rate). If the temperature is increased: The reactant particles move more quickly they have more energy the particles collide.
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product. When you heat anything, the particles move quicker. 5 ways to increase reaction speed.
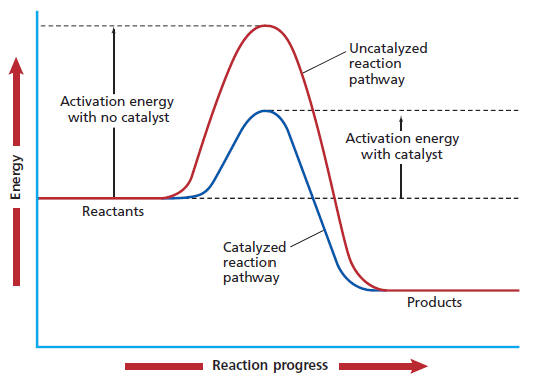
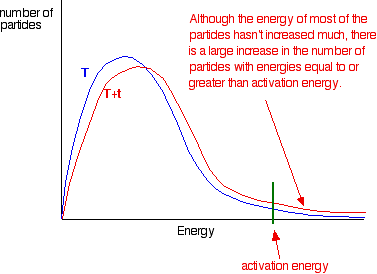
How to increase rate of reaction. This is because all reactions have an energy barrier, the activation energy. Other factors that affect the rate of a reaction.
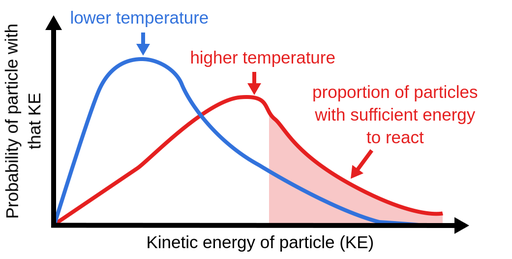
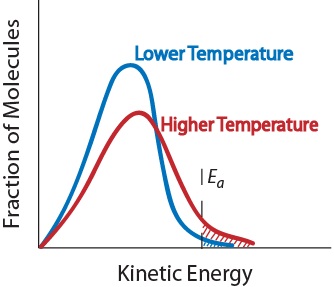
Increasing temperature, however, would result in the fastest reaction. What will increase reaction rate? An even faster reaction is set to take place if both powder and warmer temperature is used.
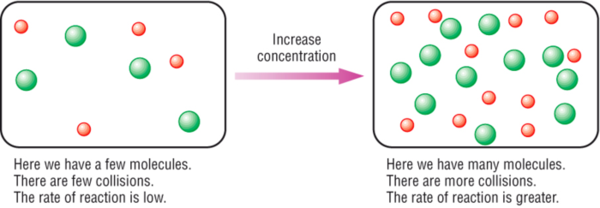
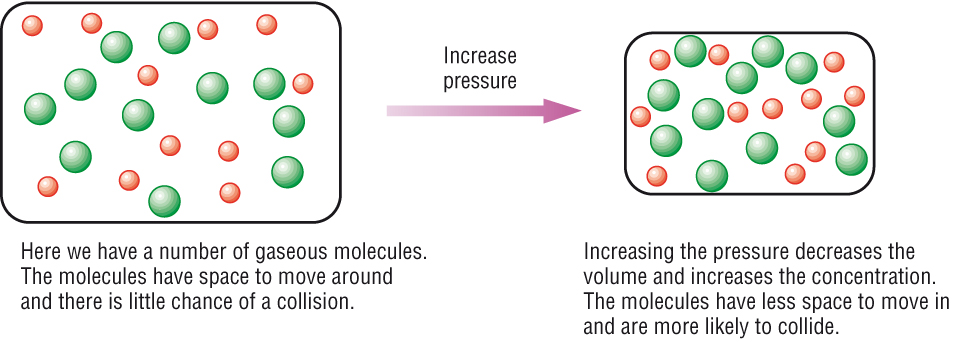
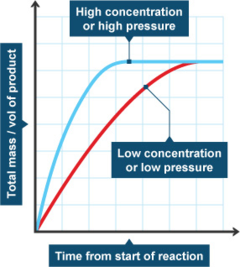
Increasing the concentration of reactants generally increases the rate of reaction because more of the reacting molecules or ions are present to form the reaction products. Collision theory of reaction rates. In general, increasing the concentration of a reactant in solution, increasing the surface area of a solid reactant, and increasing the.
Increase the temperature of the reactants. The project was done in. Why does the reaction rate change as the concentration of the reactants changes?